What Is the Average Atomic Mass of Chromium
Atomic mass of Chromium is 519961 u. Click here to get an answer to your question The average atomic mass of chromium is 5200 amu.
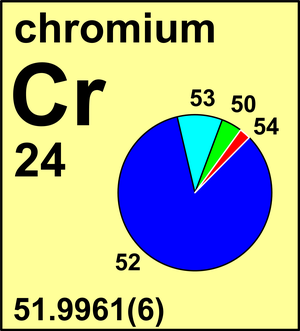
Atomic Weight Of Chromium Commission On Isotopic Abundances And Atomic Weights
435 with a mass of 4995 amu.
. 236 with a mass of 5394 amu. The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. Given the average atomic mass of an element on the periodic table and the percent natural abundance of each isotope calculate the identity of the unknown isotopeatomic mass of chromium is 51996 amu chromium-.
The average atomic mass of chromium is 51996 amu. 435 with a mass of 4995 amu. 85X 7215 849118 amu and 87X 2785 869092 amu.
This preview shows page 6 - 8 out of 10 pages. Calculate the Average Atomic Mass. 50 Cr 52 Cr 53 Cr and 54 Cr with 52 Cr being the most abundant 83789 natural abundance.
2 Show answers Another question on Chemistry. What is the mass of 30 mol of chromium. 950 with a mass of 5294 amu.
Calculate the average atomic mass of an element with two naturally occurring isotopes. What is the mass of 300 mol of chromium. The atomic mass is the mass of an atom.
Standard atomic weight Ar standardCr 519961 6 view. What is the mass of 30 mol of chromium. The average atomic mass of chromium is 5200 u.
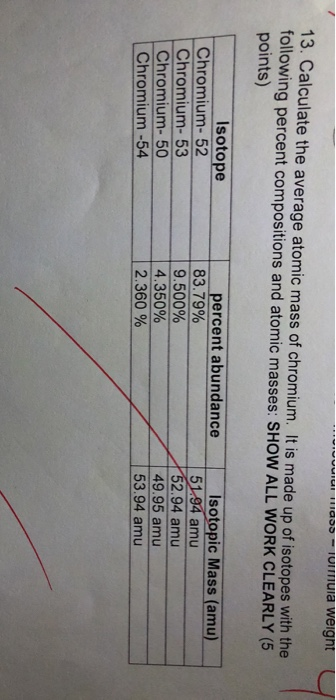
It is made up of isotopes with the following percent compositions and atomic masses. Chromium has four naturally occurring isotopes. 4345 chromium-52 8379 chromium-53 950 chromium-54 2365.
8379 with a mass of 5194 amu. Chromium 52 51941 083800 Chromium 53 52941 0095000 Chromium 54 53939 0023500 7. As always you must show all work show all units observe significant figures and box your final answers.
7 Mass Number Isotopic Mass amu Fractional Abundance. 950 of 53Cr with an atomic weight of 529407 amu. Cr-52 is the answer.
Note that the natural abundance of each isotope is given as a decimal fraction and not in percent. 53938 878 3 0023 65 7 In 1983 the Commission recommended the standard atomic weight of chromium to four decimal places Ar Cr 519961 6. The average atomic mass of chromium is 5200 amu.
Example 2 Calculate the average atomic mass of chromium. Based on the data below what is the average atomic mass of chromium. Chromium is a meta element.
On the basis of these data confirm that the average atomic. Calculate the average atomic mass of chromium. And 237 of 54Cr with an atomic weight of 539389 amu.
The average atomic mass of chromium is 5200 amu. Isotope Percentage Cr-50 43 Cr-52 838 Cr-53 95 Cr-54 24 A. Calculate the atomic weight weighted average atomic mass of chromium given the following table of data produced using a mass spectrometer.
Mechanical Engineering questions and answers. Calculate the average atomic mass of chromium to two decimal places. Given the average atomic mass of an element on the periodic table and the percent natural abundance of each isotope calculate the identity of the unknown isotopeAtomic mass of chromium is 51996 amu.
What is the average atomic mass of chromium. Atomic mass of it is 52. Rubidium is a soft silvery-white metal that has two common isotopes 85 Rb and 87 Rb.
Cr-52 because its mass number is closest to the actual atomic mass of Chromium 51996. What is this element. What is the average atomic mass.
Atomic mass of Chromium is 519961 u. 434 of 50Cr with an atomic weight of 499460 amu. Therefore this resulting atomic mass is calculated from naturally-occurring isotopes and their abundance.
Who is better messi or cristiano i need this for a chemistry class. The average atomic mass of copper is 6355 amu. Note that each element may contain more isotopes.
50 Cr is suspected of decaying by β β to 50 Ti with a half-life of more than 1810 17 years. Naturally occurring chromium 24 Cr is composed of four stable isotopes. If the only two isotopes of copper have masses of 6294 amu and 6493 amu what are the percentages of each.
8379 with a mass of 5194 amu. 950 with a mass of 5294 amu. Calculate the average atomic mass of chromium.
Chromium has the following isotopic masses and relative abundances. What is the mass of 300 mol of chromium. Meanwhile report isotope fractionation of chromium during chromate reduction resulting in δ53 Cr SRM979 values in groundwater samples as high as 58 or Ar Cr 519982 which is outside the current range of.
8379 of 52Cr with an atomic weight of 519405 amu. And 236 with a mass of 5394 amu.

Summer Work Section 7 Average Atomic Mass Of Chromium Calculation Youtube

Solved 13 Calculate The Average Atomic Mass Of Chromium It Chegg Com
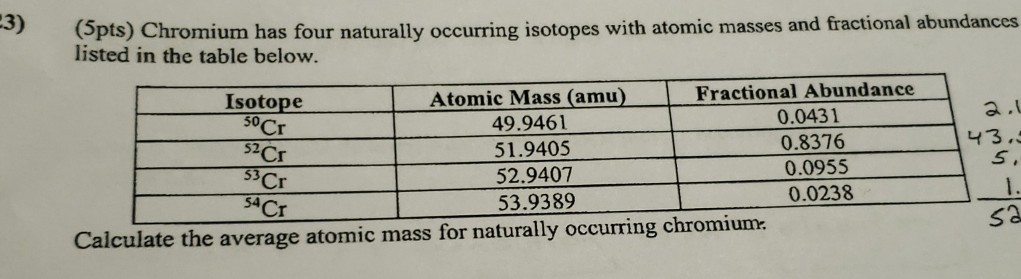
Solved 3 Pts Chromium Has Four Naturally Occurring Chegg Com
0 Response to "What Is the Average Atomic Mass of Chromium"
Post a Comment